Aztreonam
Antibiotic
General information
Aztreonam is an antibiotic. It was approved in the USA in 1986 for medical use and sold under the name e.g., Cayston®. It is used to treat infections caused by gram-negative bacteria, such as Pseudomonas aeruginosa. Nebulized forms of aztreonam are used to treat infections connected with cystic fibrosis. In relation to cystic fibrosis, our AIM tool found the data that aztreonam can improve respiratory symptoms and pulmonary function of patients.
Aztreonam on PubChem
Aztreonam on DrugBank
Aztreonam on Wikipedia
Synonyms
AZLI, aztreonam lysinate, aztreonam lysine
Marketed as
Dietary sources
N/A
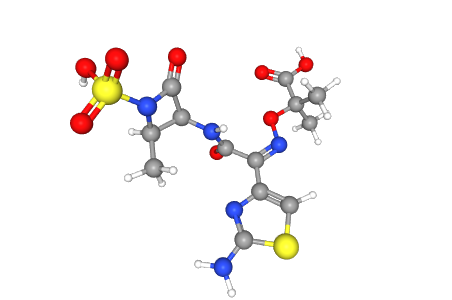
C19H31N7O10S2
Drug-Mutation Relation
results for 1078delT / 3199del6
See all data on AztreonamTreats
| Mutation | Link | Tested on | Impact factor | Notes |
|---|---|---|---|---|
| General effect | The effects of inhaled aztreonam on the cystic fibrosis lung microbiome | humans | 9.13 | |
| General effect | Inhaled aztreonam lysine for chronic airway Pseudomonas aeruginosa in cystic fibrosis. | humans | 9.79 | Tested in combination with tobramycin. |
| General effect | An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. | humans | 2.24 | |
| General effect | Efficacy and safety of inhaled aztreonam lysine for airway pseudomonas in cystic fibrosis. | humans | 6.36 | |
| General effect | Aztreonam for inhalation solution (AZLI) in patients with cystic fibrosis, mild lung impairment, and P. aeruginosa. | humans | 3.19 | |
| General effect | Open label study of inhaled aztreonam for Pseudomonas eradication in children with cystic fibrosis: The ALPINE study. | humans | 3.85 |
Does not treat
| Mutation | Link | Tested on | Impact factor | Notes |
|---|
